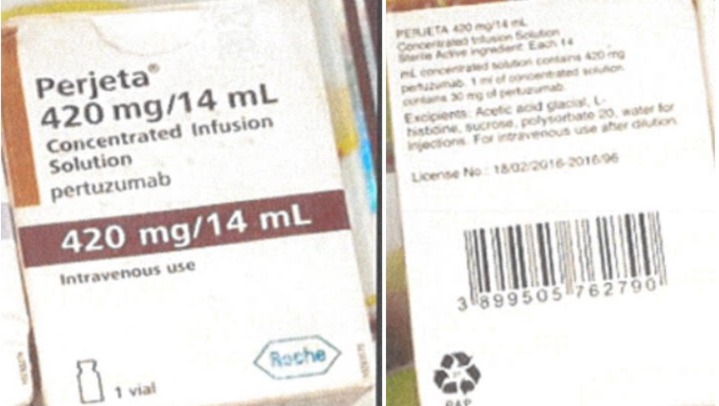
The National Agency for Food and Drug Administration Control (NAFDAC), has alerted healthcare providers and the public of a report of counterfeit Perjeta units being in circulation in Nigeria.
NAFDAC, in a release on its official website stated, “Marketing Authorization Holder (MAH) Roche received a complaint from a pharmacist reporting two units of suspected counterfeit Roche products, Perjeta 420 mg/14mL.
“The Kaiseraugst/Switzerland Quality Control organisation investigated the complaint sample pictures. As the batch number is not a genuine Roche batch number, a comparison against a batch corresponding retain sample was not possible. Since the sample may mimic the Roche common make-up EFA, a corresponding retain sample was used for comparison.”
However, significant differences were found about the packaging materials as the folding box (including the Tamper Evident label) does not correspond to Roche Perjeta’s genuine packaging material.
“Batch Trace: The lot tracing was impossible because the lot number imprinted is not a genuine Roche batch number for Perjeta,” the statement noted.
It added that the chemical analysis was not possible as the physical complaint sample was not available for return.
According to NAFDAC, investigation on the provided pictures revealed clear evidence of counterfeit packaging material.
“Perjeta 420mg Injection is used to treat breast cancer when other medicines have failed to show significant improvement.
“It helps to stop cancer growth and further spread and relieves the symptoms of breast cancer such as breast lumps, bloody discharge from the nipple, or changes in the shape or texture of the breast,” NAFDAC stressed.
Healthcare professionals and consumers are therefore advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office.
Such complaints are also asked to me made through call or email to NAFDAC at 0800-162-3322, and [email protected].